Research
THERAPY RESISTANCE
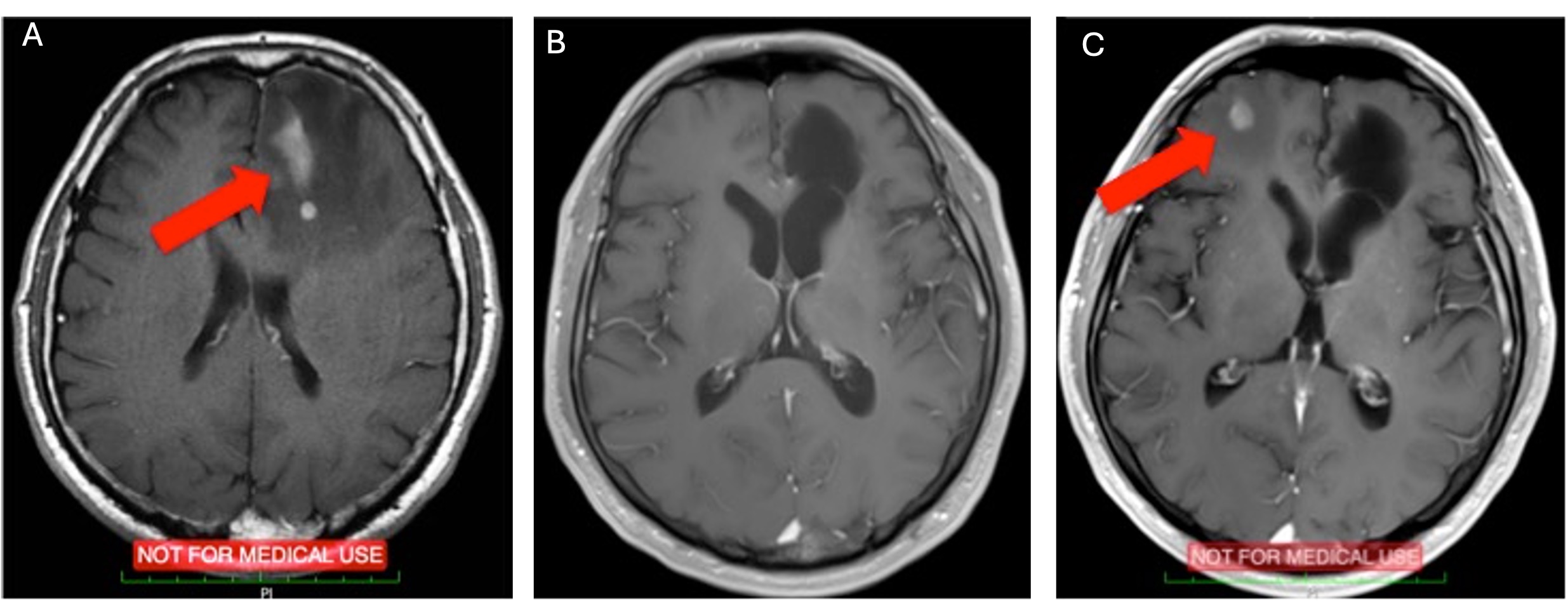
While most therapeutic agents are directed to keep tumor cell proliferation under control, very few agents aim to reactivate the dormant or inactive apoptotic pathways in tumor cells. After the initial discovery of TRAIL (Apo2L) as an apoptotic molecule, its promise as a therapeutic agent has become evident by its tumor-specific action. However, many tumor cells, including glioblastoma, are either innately TRAIL resistant or acquire resistance with mechanisms that remain poorly understood. In addition to apoptotic resistance, these resistance mechanisms may also be related to non-genetic factors originating from intracellular dynamics. Moreover, some survival pathways are effective in TRAIL resistance. Understanding these resistance mechanisms will open new horizons in therapeutic approaches targeting cancer cells.
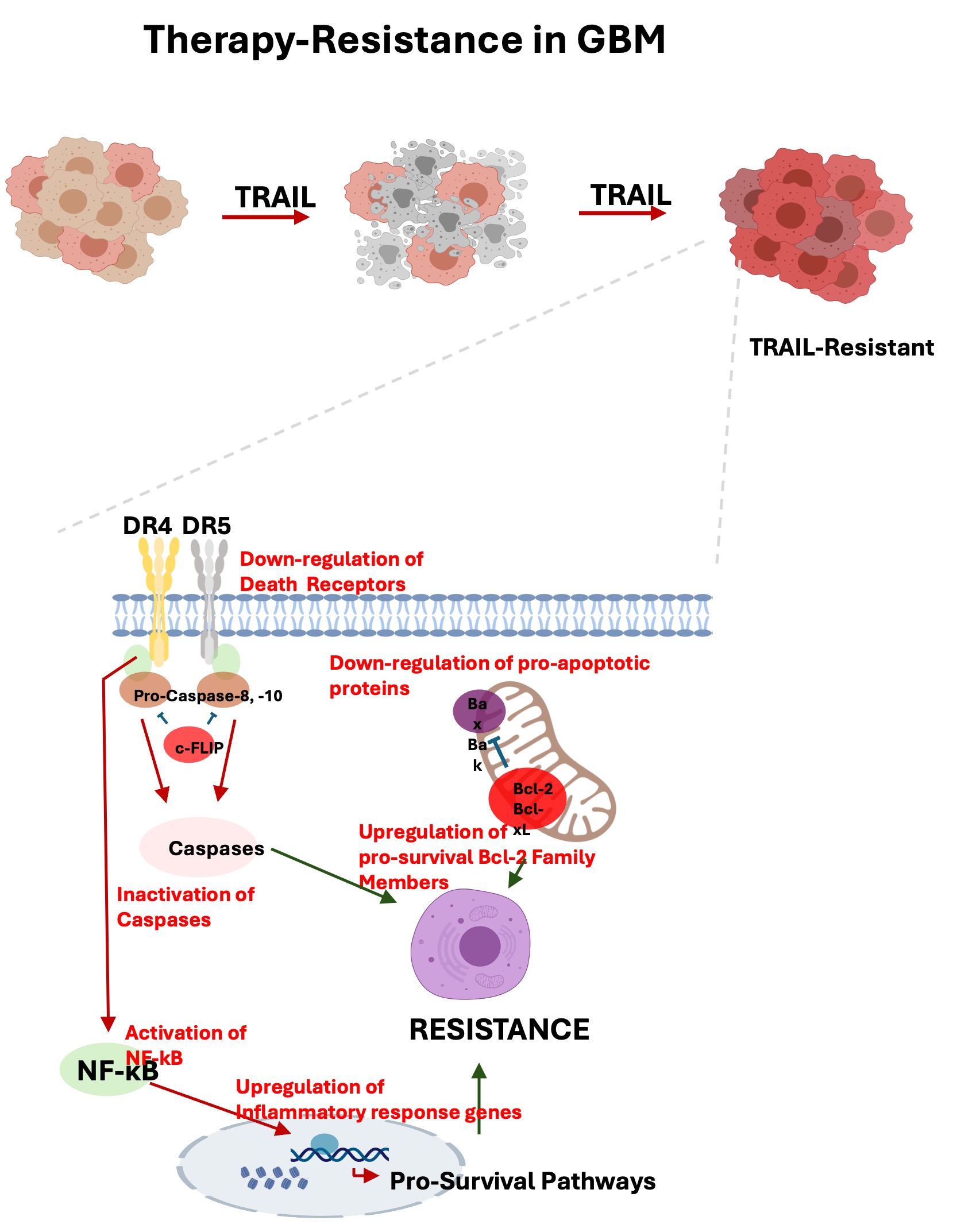
Cingöz et. al., 2021.
TUMOR METABOLISM
Glioblastoma, like many other tumor types, primarily prefers glucose in energy metabolism. One of the cornerstones of GBM metabolism is aerobic respiration (OxPhos). Although there are many mitochondrial abnormalities in tumors, according to many researchers, mitochondria and OxPhos mechanisms are normal and regular in GBM tissues (Chinopoulos and Seyfried, 2018). Glucose and glutamine are required for high antioxidant capacity in tumor cells, which causes chemotherapy and radiotherapy resistance. Glutamine is also one of the key substrates of the citric acid cycle. However, it is known that in most tumors the metabolic pathway is reprogrammed for sufficient production of biomolecules necessary for nucleotide, lipid and amino acid synthesis, which is necessary and important for the rapid proliferation of malignant cells (Heiden et al., 2009; DeBerardinis and Chandel, 2020). In gliomas, mitochondrial reprogramming has been demonstrated for adaptation to the microenvironment, especially in hypoxic conditions. It is known that HIF-1 increases the uptake of glutamine, the second important substrate in the growth of cancer cells. Glutamine is also the nitrogen source of nucleotides and non-essential amino acids. Additionally, glutamine participates in the Krebs cycle by converting into glutamate and α-ketoglutarate, and as a result, it is converted into lactate (Domènech et al., 2021). The main changes in mitochondrial metabolism of GBM cells consist of changes in OxPhos and secondary metabolic changes related to the TCA cycle, mitochondrial membrane potential, and mitochondrial dynamics (Wu, Ho and Lu, 2021).
To this end, we are utilizing noninvasive imaging techniques, genome editing tools and in vitro and in vivo high throughput screening strategies to identify novel mechanisms of GBM cell therapy and tumor metabolims.